- Afhalen na 1 uur in een winkel met voorraad
- Gratis thuislevering in België vanaf € 30
- Ruim aanbod met 7 miljoen producten
- Afhalen na 1 uur in een winkel met voorraad
- Gratis thuislevering in België vanaf € 30
- Ruim aanbod met 7 miljoen producten
Zoeken
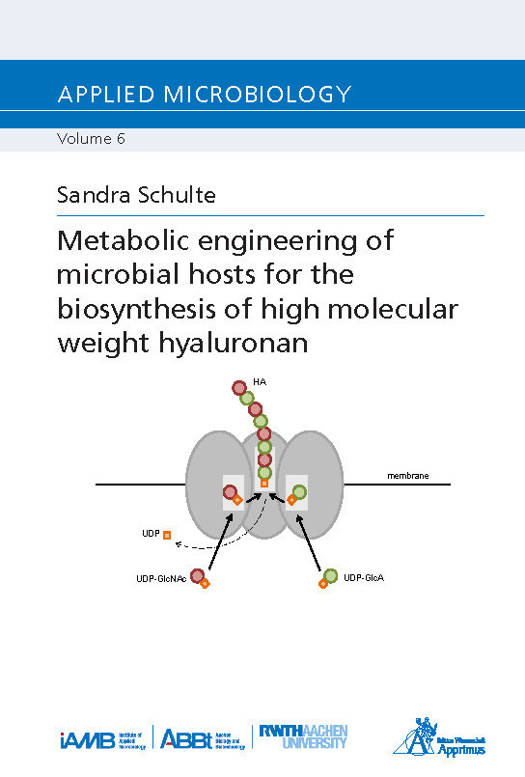
Metabolic engineering of microbial hosts for the biosynthesis of high molecular weight hyaluronan
Sandra Schulte
€ 38,45
+ 76 punten
Omschrijving
Many recombinant bacteria for hyaluronan (HA) production have been reported during the last years. They exhibit significant advantages when compared to extraction of HA from animal tissues or fermentation of pathogenic bacteria. Less organic solvents are required, the host organisms are not toxic and do not degrade HA, and there is no need for extensive downstream-processing. Nevertheless, all these catalytic systems share one significant limitation: a low molecular weight (MW). As a high MW is of great importance, it is desirable to establish an industrially feasible production process for this field. Therefore, the aim of this study was the development of a recombinant microbe to produce HA with a MW of 2 MDa and more. Two strategies were followed: 1. Saccharomyces cerevisiae is a well-established industrial workhorse and genetic tools are widely available. Using S. cerevisiae as production host allows the investigation of HA synthases originating from higher eukaryotes, the expression of which might be quite complicated using a prokaryotic host. Also, a lot is known about the physiology of S. cerevisiae, which will simplify manipulation of its genome by deleting undesired pathways and integrating new genes. A synthetic pathway for precursor supply will further facilitate HA synthesis. 2. Different Streptococcus species are known to produce HA of different MW. As the determinants for the MW of HA have not yet been fully elucidated, a closer look will also be taken at several streptococcal synthases to see whether sequence differences result in MW differences. Two new streptococcal HA synthases from Streptococcus parauberis and Streptococcus iniae were also included. Moreover, it was investigated how the synthases behave under HA production conditions in recombinant Lactococcus lactis and whether that performance differs in vitro.
Specificaties
Betrokkenen
- Auteur(s):
- Uitgeverij:
Inhoud
- Aantal bladzijden:
- 144
- Taal:
- Engels
- Reeks:
Eigenschappen
- Productcode (EAN):
- 9783863596293
- Uitvoering:
- Paperback
- Afmetingen:
- 148 mm x 210 mm
- Gewicht:
- 276 g
Alleen bij Standaard Boekhandel
+ 76 punten op je klantenkaart van Standaard Boekhandel
Beoordelingen
We publiceren alleen reviews die voldoen aan de voorwaarden voor reviews. Bekijk onze voorwaarden voor reviews.